acf domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /data/2/5/3/144/5492796/user/6572858/htdocs/wp-includes/functions.php on line 6121Enables rapid and accurate transperineal image-guided (MR/CT) needle placement. Transperineal access substantially reduces the risk of sepsis associated with transrectal biopsies. The device further allows the diagnosis and treatment of metastatic lesions not available with transrectal procedures. The streamlined workflow provides the option of combining targeted biopsies and treatments such as cryoablation in one session within the MRI suite. Our solution simplifies the current workflow, improves targeting accuracy, and significantly reduces the number of MRI scans needle to verify needle placement resulting in a reduced procedure time.



The chances of infection following transrectal (TR) prostate biopsy is relatively high ranging from 0.1% to 7.0% resulting in a high chance of sepsis ranging from 0.3% to 3.1%. This risk is increasing because antibiotic-resistant bacteria are becoming more common. The cost of treating sepsis is high approaching of hundreds of millions per year in the United States and still increasing.
Internationally, the practice of using the transperineal approach (TP) has been growing. Patients who undergo both TR and TP preferred TP over TR 42% to 31%. A 2019 review concluded that while TP biopsy offers the same diagnosis accuracy of TR prostate biopsy, the risk of infection and rectal bleeding is significantly lower making it a much safer alternative. The recommendation was to perform TP biopsy whenever possible. In January 2021, the European Association of Urology stated, “Available evidence highlights that it is time for the urological community to switch from a transrectal to a transperineal approach despite any possible logistical challenges”.

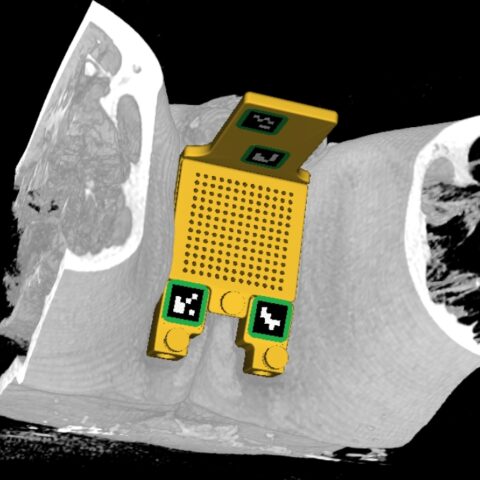
The device can be used for any procedure which involves transperineal needle placement. This includes 12-core biopsies, targeted biopsies, prostate cryoablation, Seminal Vesicle ablation, Pelvic Lymph node ablation, etc.
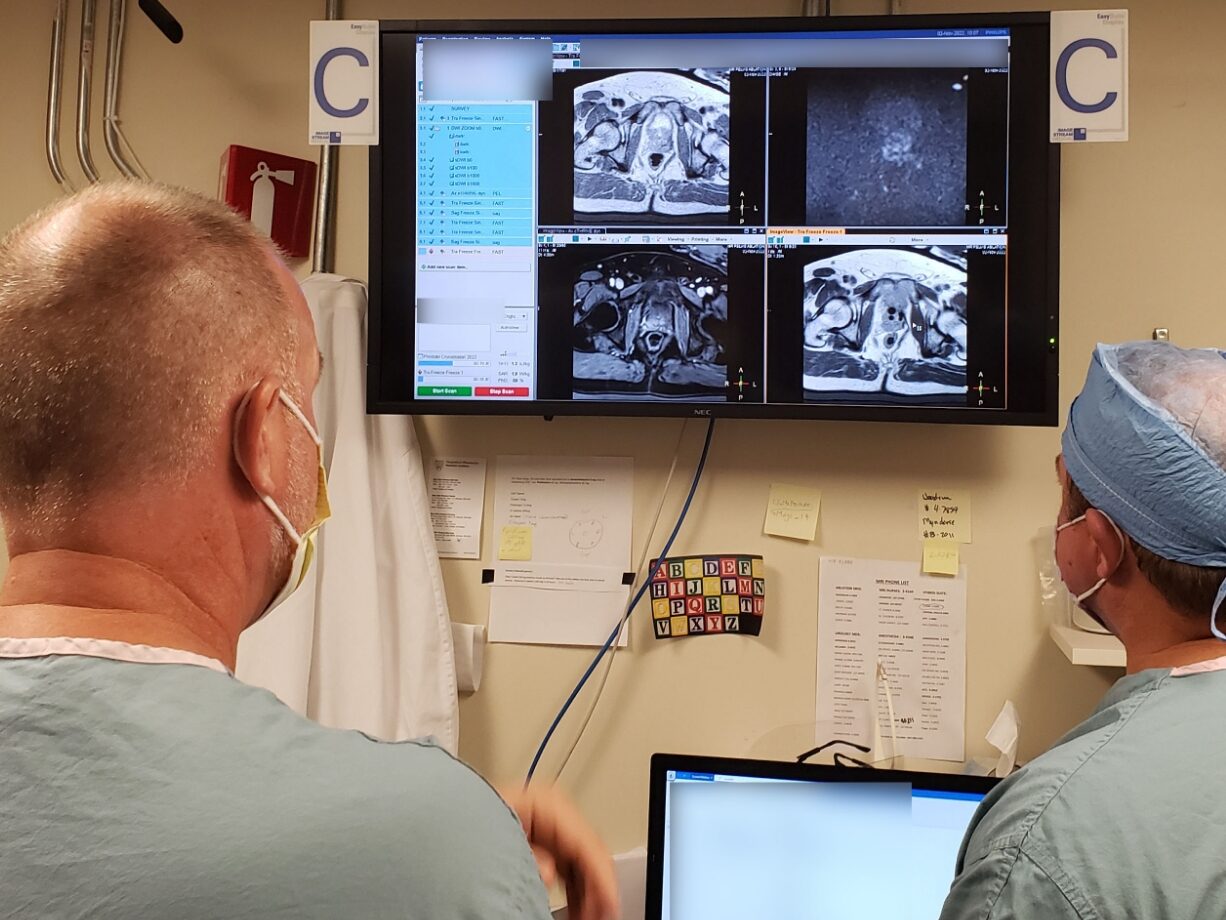
The SCENERGY platform offers a planning solution which can be used with CLEAR GUIDE SteriGRID® that automatically localizes the grid and guides the insertion. The first step is to acquire an initial planning/diagnostic scan with the grid which is sent to our device. Our software automatically overlays grid holes on the CT/MR images. The physician can browse through images to both select a target and review the path of the needle. Following the guidance, the physician inserts the needle through the highlighted needle guide hole. A verification scan ensures the success of needle placement.
Superior quality of MRI for soft tissue is key for targeting and needle placement in prostate as well as other procedures. However, lack of a guidance device has traditionally limited MRI into a diagnostic modality where the images are obtained prior to any biopsies or intervention. By providing guidance and reducing the required scanner time, our device unlocks widespread adoption of in-suite MRI interventions. CLEAR GUIDE SCENERGY – TP also works for CT imaging for cases where MRI is not an option or when CT is preferred.
We have conducted a clinical trial in collaboration with Mayo Clinic at Rochester (https://clinicaltrials.gov/study/NCT05573048) where the device was successfully used for biopsy and cryoablation procedures. According to our collaborators, this guidance device was fast and accurate and offered a superior workflow. A clinical publication is in the works. Prior to this study, we conducted a phantom study the results of which can be found here: https://doi.org/10.1117/12.2653979.


Clear Guide Medical is a leader in next-generation navigation technology for image fusion and augmented reality. Our vision-based approach has been introduced in the CLEAR GUIDE SCENERGY with Instrument Guidance and Image Fusion.
Applying computer vision to medical needs, our products make minimally invasive interventions like biopsies, ablations, pain injections, and peripheral nerve blocks efficient and cost-effective.

“As a secondary endpoint, the ClearGuide transperineal guidance device was successfully used to target and treat metastatic prostate lesions. Compared to the current alternatives, the device offers a superior workflow which is easy to follow. This workflow eliminates the need for manual segmentation of grid fiducials in MRI volumes since the markers are automatically detected.”
